Photon
A photon is the basic quantum of electromagnetic energy, spanning the entire electromagnetic spectrum-from low to high energies and from long to short wavelengths. This includes radio waves, microwaves, visible light, ultraviolet radiation, X-rays, and gamma rays. Most electronic devices are enclosed in opaque packaging (e.g., plastic, ceramic, or metal), so photons (light) in the visible range generally pose no concern. However, high-energy photons such as X-rays and gamma rays can penetrate these materials and affect device operation. For space electronics, charged particles (protons, heavy ions, electrons) and neutrons are usually the dominant concern; nevertheless, in industrial and medical settings X-rays and gamma rays are primary radiation sources, typically spanning ~10 keV to 1,000 keV. In this range, the photoelectric effect tends to dominate at lower energies and in high-Z materials, while Compton scattering becomes increasingly important at intermediate energies; above ~1.022 MeV, pair production can dominate. Because photons are electrically neutral, their interactions with matter differ from those of charged particles. The three principal photon-matter interaction mechanisms are summarized below.
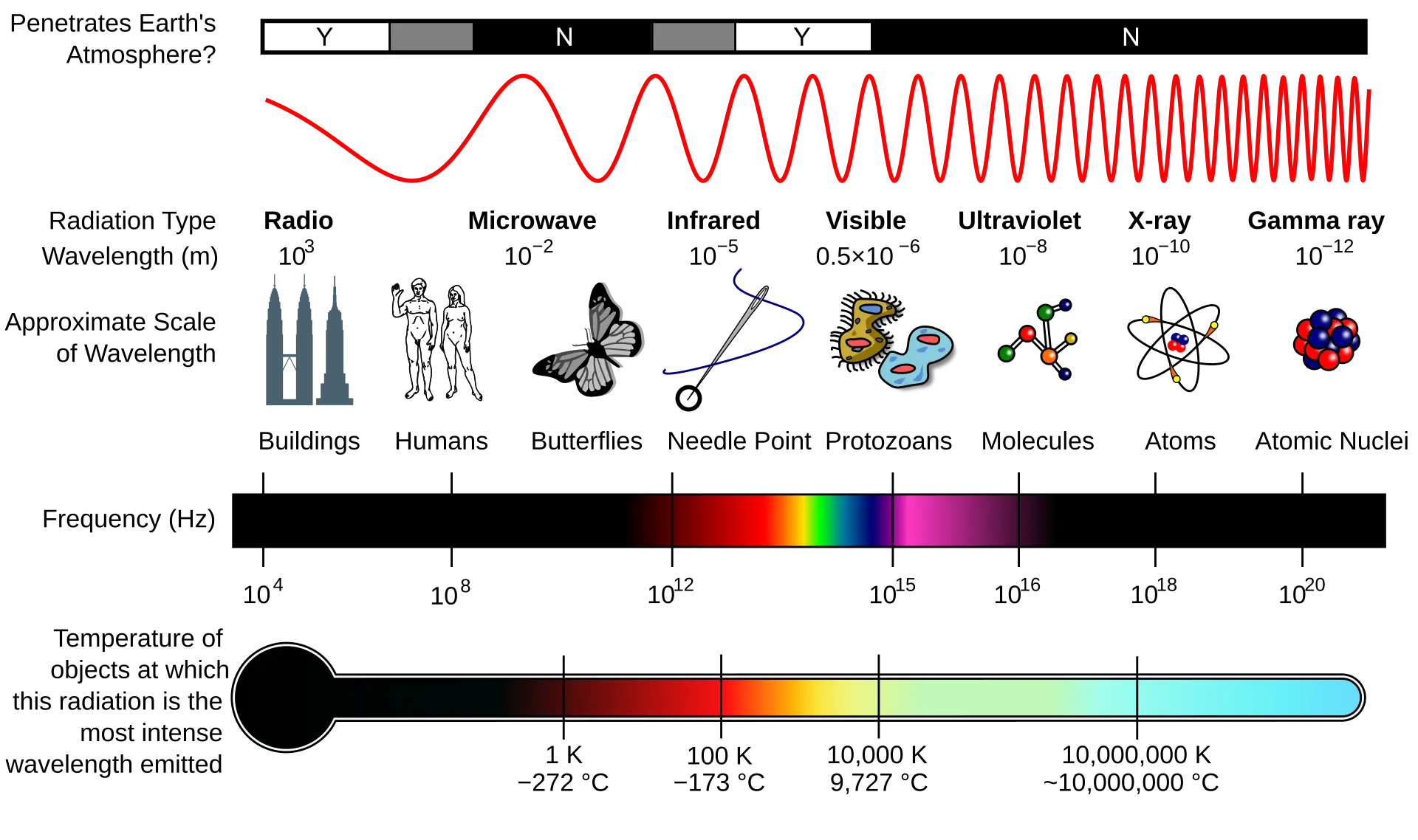
Diagram illustrating the electromagnetic spectrum
Image source: EM Spectrum Properties edit.svg, created by Inductiveload & NASA (adapted from NASA’s File:EM Spectrum3-new.jpg).
Licensed under CC BY-SA 3.0 and GNU Free Documentation License 1.2+.
You are free to use and modify this image with attribution and ShareAlike terms.
1. Photoelectric Effect
When a photon with sufficient energy interacts with a semiconductor, it can liberate an electron from the valence band (or from a bound atomic state), producing a photoelectron and leaving behind a positively charged hole.
In the X-ray regime (tens to hundreds of keV), inner-shell electrons can be ejected; subsequent relaxation of outer-shell electrons may emit characteristic X-rays that are element-specific.
In the visible/UV range, photons primarily excite valence-band electrons into the conduction band, generating electron-hole pairs.
The photoelectric effect is an inelastic interaction in which the photon’s energy is absorbed. If the photon energy is too low to generate an electron-hole pair, the material is effectively transparent at that wavelength.
2. Compton Scattering
In Compton scattering, a photon transfers part of its energy to an electron, producing a lower-energy scattered photon and a recoil electron. Depending on the transferred energy, the electron may be excited to a higher state or fully freed, after which it can further interact and generate additional ionization.
3. Pair Production
Pair production is a main high-energy gamma-ray loss mechanism. A photon interacting in the field of an atomic nucleus can transform into an electron-positron pair, provided the photon energy exceeds the combined rest-mass energy of the pair (> 1.022 MeV). Any excess energy appears as kinetic energy shared by the particles. The probability of pair production increases with photon energy and with the atomic number (Z) of the material; below the threshold, the process cannot occur.
Related Articles
- Korean Radiation Test Facilities
- Energy Transfer Mechanisms of Electrons
- What is a Heavy-Ion Beam?
- Energy Transfer Mechanisms of Protons and Neutrons
- Accidents Caused by Soft Errors
- Energy Transfer Mechanisms of Ions
- How Was SEU Discovered? A Historical Insight into Radiation-Induced Failures in Electronic Circuits


